An acid is a chemical species that donates protons and hydrogen ions and accepts electrons. The acidity of a liquid depends on the concentration of H+ ions only. The higher the concentration of H+ ions, the lower the PH it develops and gets high acidity. The origin of the word acid took place from the Latin word acidus or acere, which refers to sour. That is, a mixture with a ph of less than seven and sour in taste is acid. Now, there are different types of acids with their unique formula and characteristics. Here are the sources of these acids that you must know. But let’s first get the basics clear with:
Characteristics of an Acid
- An acid has a PH of less than seven i.e.; below neutral
- The evidence of the low PH of acids is the conversion of blue litmus paper with red
- The acids usually taste sour like vinegar
- The acids have an odor that of burning sensation
- The texture of acid is sticky and slippery
- Acids have the tendency to react with metals to produce hydrogen gas
Different Sets of Acids with the Definition
Arrhenius Acid
As per this definition, an acid is a substance that adds Hydronium ions to the water, thus increasing its concentration.
Bronsted-Lowry Acid
An acid acts as a proton donor. Any substance that deprotonates itself into typical acids, plus amines, or alcohol is an acid.
Lewis Acid
It is a compound that accepts an electron pair and forms a covalent bond.
Now acids when defined categories into two:
Strong Acids – The acid that completely dissociates into its ion when in water
E.g.: Hydrochloric Acid
Weak Acids – The acid that partially dissociates into its ions when in water
E.g.: Acetic Acid
Types of Acids with Examples
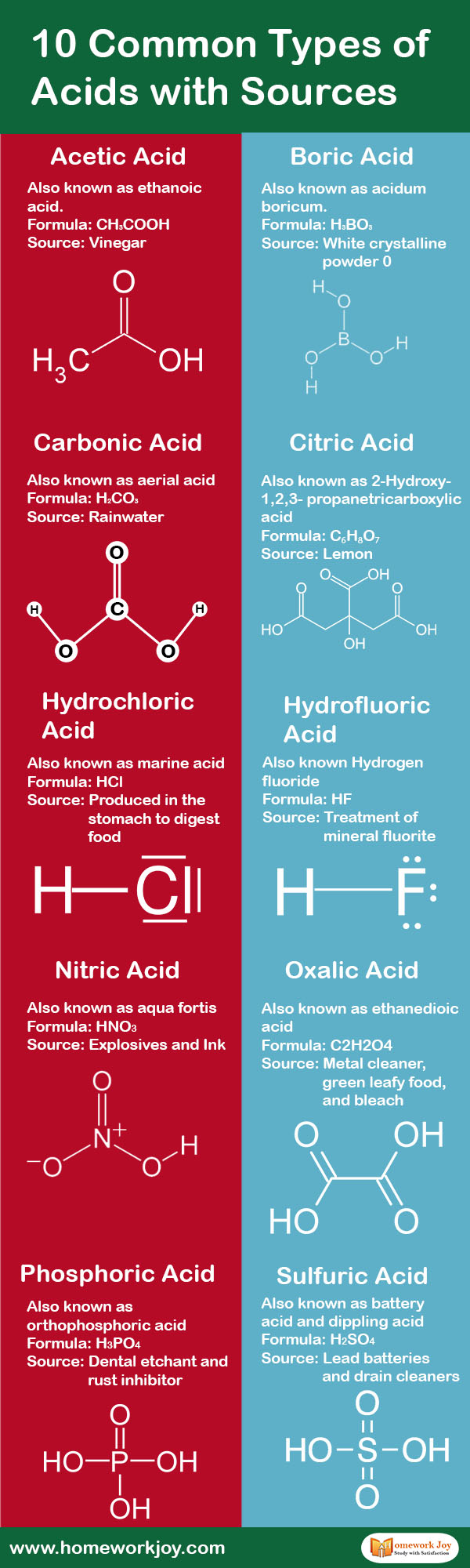
Acetic Acid
Also known as ethanoic acid
Formula: CH3COOH
Source: Vinegar
Boric Acid
Also known as acidum boricum
Formula: H3BO3
Source: White crystalline powder
Carbonic Acid
Also known as aerial acid
Formula: H2CO3
Source: Rainwater
Citric Acid
Also known as 2-Hydroxy-1,2,3-propanetricarboxylic acid
Formula: C₆H₈O₇
Source: Lemon
Hydrochloric Acid
Also known as marine acid
Formula: HCl
Source: Produced in the stomach to digest food
Hydrofluoric Acid
Also known Hydrogen fluoride
Formula: HF
Source: Treatment of mineral fluorite
Nitric Acid
Also known as aqua fortis
Formula: HNO3
Source: Explosives and Ink
Oxalic Acid
Also known as ethanedioic acid
Formula: C2H2O4
Source: Metal cleaner, green leafy food, and bleach
Phosphoric Acid
Also known as orthophosphoric acid
Formula: H3PO4
Source: dental etchant and rust inhibitor
Sulfuric Acid
Also known as battery acid and dippling acid
Formula: H2SO4
Source: Lead batteries and drain cleaners
If you need more help related to similar topics, take instant online homework help from our professors.