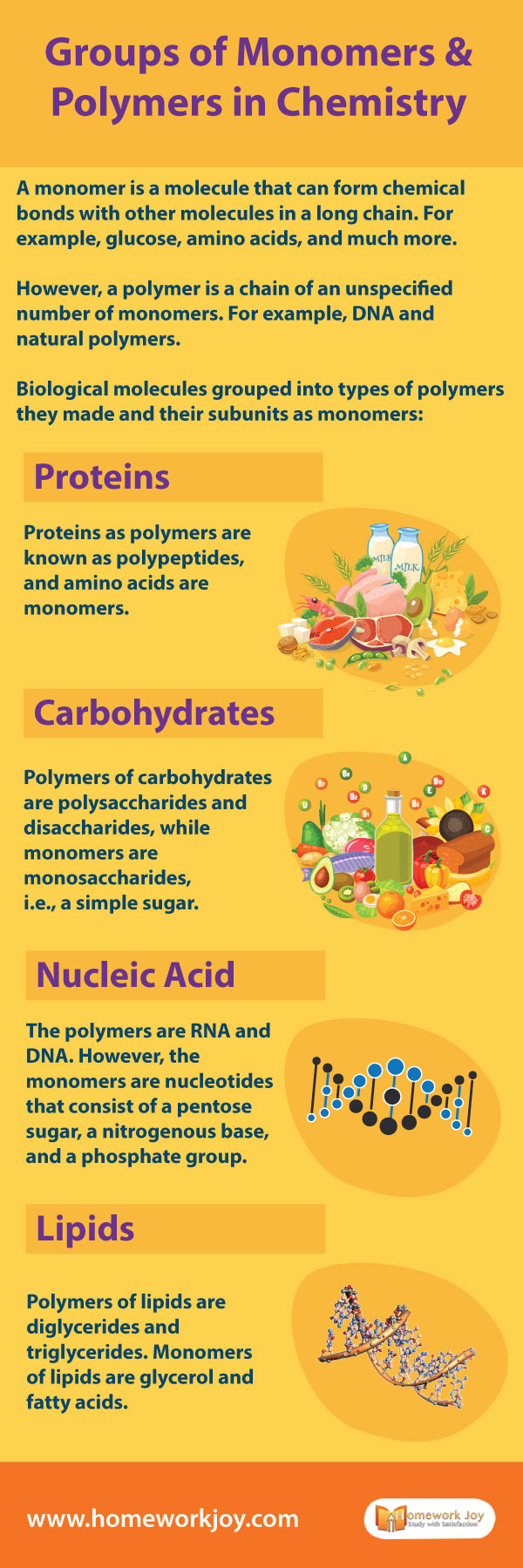
A monomer is a molecule that can form chemical bonds with other molecules in a long chain—for example, glucose, amino acids, and much more. However, a polymer is a chain of unspecified monomers—for example, DNA and natural polymers. Biological molecules are grouped into types of polymers they made and their subunits as monomers. In this tutorial, you’ll learn about the groups of monomers and polymers in
Also read:-
What are Monomers With Examples?
The word monomer comes from mono- (one) and -mer (part). Monomers are small molecules, joined together in a repeating fashion to form complex molecules called polymers. These small molecules come together to form polymers by forming chemical bonds or binding supramolecular through a process called polymerization. When a dozen monomers are bound in groups, they form oligomers. E.g., collagen and liquid paraffin. In short, monomers are building blocks of polymers.
Examples:-
Vinyl Chloride – polymerizes into polyvinyl chloride
Glucose – polymerizes into starch, cellulose, laminarin, and glucans
Amino Acids – polymerize into peptides, polypeptides, and proteins
Note
Glucose is the most abundant form of natural monomer.
What are Polymers With Examples?
The word polymers are made from poly – (many) and -mer (part). Polymers are natural or synthetic macromolecules comprised of repeating units of a smaller molecule called monomers. Under the term polymers, you’ll find plastics, cellulose, amber, and natural rubber. Based on the monomeric subunits, the lower molecular weight compounds are distinguished as dimers, trimer, tetramer, pentamer, hexamer, octamer, nonamer, and decamer dodecamer, etc.
Examples:-
Examples of polymers include:
- plastics such as polyethylene
- silicones such as silly putty
- biopolymers such as cellulose and DNA
- natural polymers such as rubber and shellac
Groups of Monomer and Polymers
Now that you know the basic definition of the terms monomer and polymer, here are the main groups categorized under them. Here are the classes of biological molecules grouped into the types of polymers they form and the monomers acting as the subunits.
Proteins
Proteins as polymers are known as polypeptides, and amino acids are monomers.
Carbohydrates
Polymers of carbohydrates are polysaccharides and disaccharides, while monomers are monosaccharides, i.e., a simple sugar.
Nucleic Acid
The polymers are RNA and DNA. However, the monomers are nucleotides that consist of a pentose sugar, a nitrogenous base, and a phosphate group.
Lipids
Polymers of lipids are diglycerides and triglycerides. Monomers of lipids are glycerol and fatty acids.
Short Note on Polymerization
If you’re wondering how the polymers are formed from their monomers, here is a short note on the process. Polymerization is the process of bond formation between the smaller monomers and the polymers through a covalent bond. During this process, the monomers lose their chemical groups and join together. Meanwhile, in the case of biopolymers of carbohydrates, this is a dehydration process, resulting in water formation.
If you need help with more such chemistry topics, you can seek instant online assignment help from our experts. We have a team of professionals available for you 24*7.