Have you ever thought about how reactions take place? It is only due to these laws of thermodynamics. You have heard about this term. But still, you must be in a state of confusion. To avoid this confusion, here we’ll discuss three laws of thermodynamics about a system and its surroundings. These laws express scientific facts and define physical quantities like temperature, heat, thermodynamic work, and entropy.
But before knowing these laws of thermodynamics, let’s understand thermodynamics first.
What is Thermodynamics?
A branch of science that deals with the study of heat and temperature are what we call as thermodynamics. It relates to other forms of energy. Also, it applies to a variety of science and engineering such as chemical, physical and mechanical engineering. For improving the efficiency of steam engines, thermodynamics was developed.
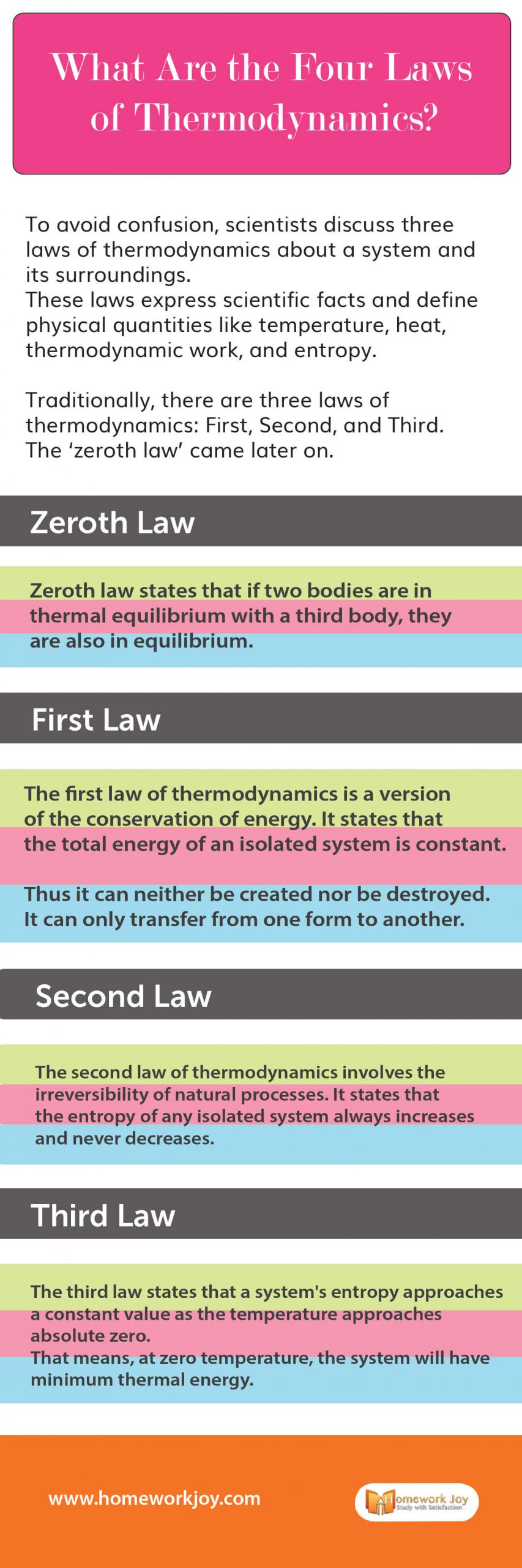
Traditionally, there three laws of thermodynamics: First, Second, and Third. The ‘zeroth law’ came later on. Besides this, it involves Boyle’s Law and Charles Law, along with various properties. Let’s know more about these laws.
Zeroth Law
Zeroth law states that if two bodies are in thermal equilibrium with a third body, they are also in equilibrium.
First Law
The first law of thermodynamics is a version of the conservation of energy. It states that the total energy of an isolated system is constant.
Thus it can neither be created nor be destroyed. It cn only transfer from one form to another.
Second Law
The second law of thermodynamics involves the irreversibility of natural processes. It states that the entropy of any isolated system always increases and never decreases.
Third Law
The third law states that a system’s entropy approaches a constant value as the temperature approaches absolute zero.
That means, at zero temperature, the system will have minimum thermal energy.
Applications of Thermodynamics
Here are some common applications of thermodynamics:
- In a crowded room, everyone starts sweating. The body starts calling down by transferring heat to the room. Thus this case is the explain first and second law of thermodynamics. The heat energy is not lost. It is transferred by attaining equilibrium with maximum entropy.
- When we take out chilled water from the refrigerator, it cools down to room temperature after some time. It attains room temperature by absorbing atmospheric heat. Again, it happens due to the first and second law of thermodynamics.
Thus learning these las of thermodynamics is essential to understand different reactions in chemistry. If you need more help, get instant online assignment help from our experts 24*7.