All of us have seen states of matter in our everyday life. But do you know what states of matter of what is matters is? So don’t worry if you don’t know. Here we’ll discuss these things in brief. So the matter is “material substance that constitutes the observable universe and, together with energy, it forms the basis of all phenomenon.” But today we’ll learn about these different states of matter from a scientific point fo view.
Also, read some of our latest posts before moving ahead:
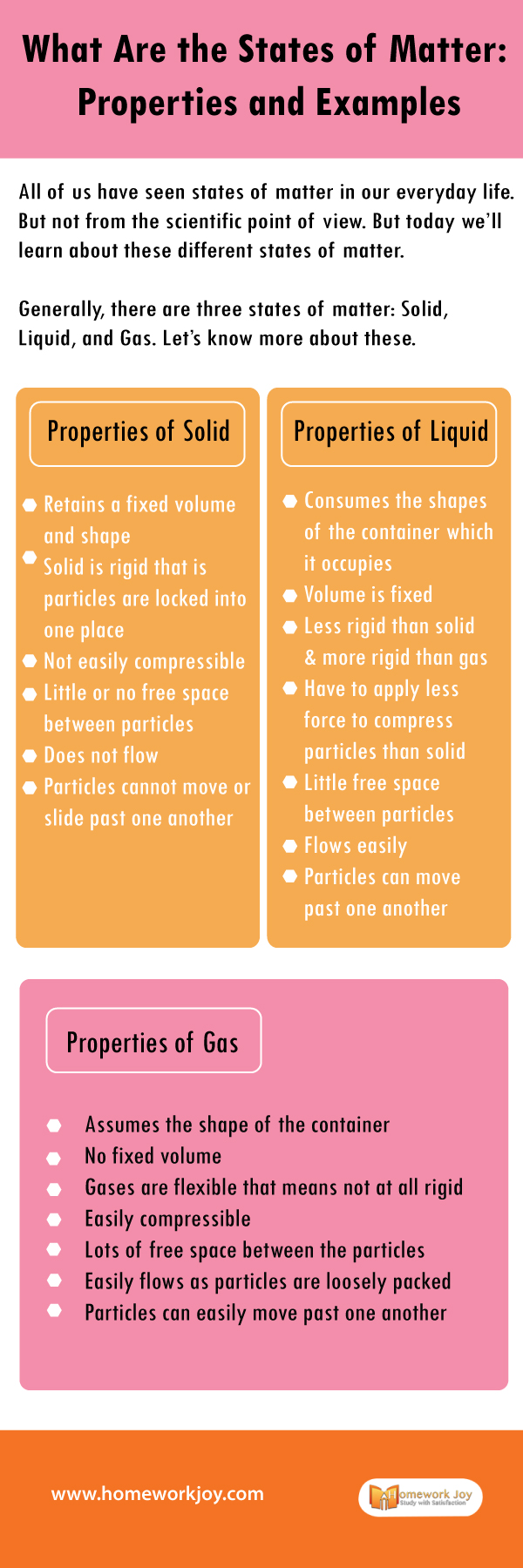
Generally, there are three states of matter: Solid, Liquid, and Gas. Let’s know more about these.
Properties of Solid
-
Retains a fixed volume and shape
-
Solid is rigid that is particles are locked into one place
-
Not easily compressible
-
Little or no free space between particles
-
Does not flow
-
Particles cannot move or slide past one another
Properties of Liquid
-
Consumes the shapes of the container which it occupies
-
Volume is fixed
-
Less rigid than solid and more rigid than gas
-
Have to apply less force to compress particles than solid
-
Little free space between particles
-
Flows easily
-
Particles can move past one another
Properties of Gas
-
Assumes the shape of the container
-
No fixed volume
-
Gases are flexible that means not at all rigid
-
Easily compressible
-
Lots of free space between the particles
-
Easily flows as particles are loosely packed
-
Particles can easily move past one another
How does Matter Move from One State to Another?
As energy transforms from one state to another, similarly energy from matter causes physical changes phases from one to another. For example, adding heat to water changes it into water vapours. Thus in this way, liquid changes into a gaseous state. These physical changes can also be caused by pressure and motion.
Melting and Freezing
When heat is applied to a solid, it changes its state as its particles begin to vibrate and move farther apart. The state in which the substance reaches to a combination of temperature and pressure is called its melting point.
Moreover, when the liquid is cool down, its particles slow down and begin to settle in one location within the substance. The state in which a substance reaches enough temperature at a certain pressure, it is known as the freezing point. In this state, the liquid becomes solid.
Thus these were some essential topics that you must know to understand different states of matter. To know more, get instant science assignment help from us.