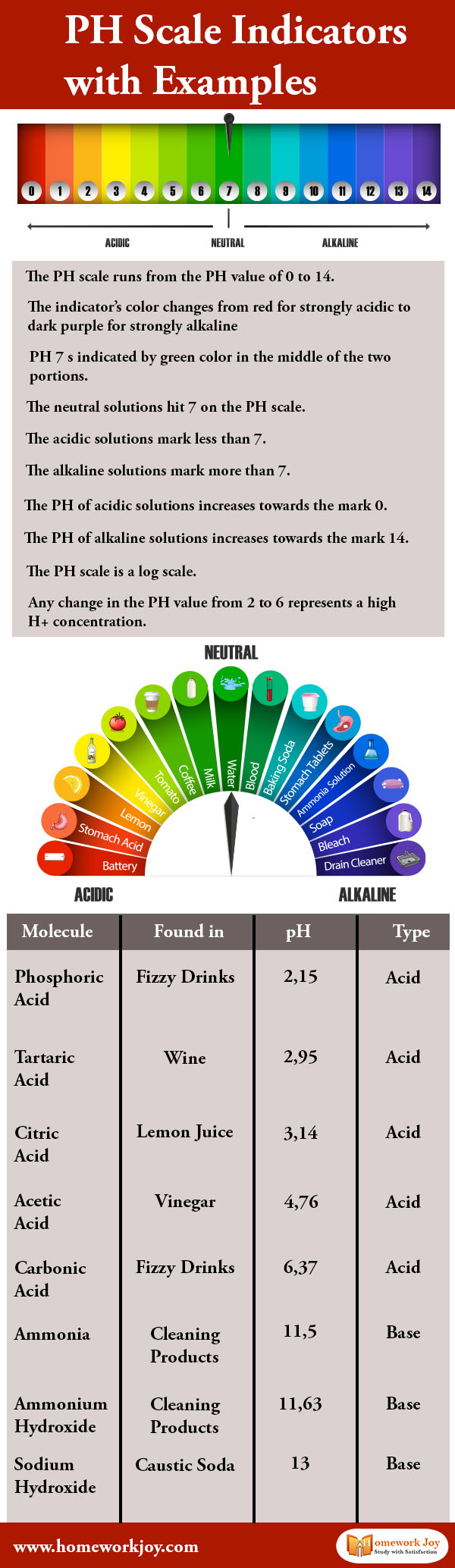
In the pH scale, the P stands for “potential,” and the h stands for “hydrogen.” Together it means “potential of hydrogen,” which measures the acidity and basicity of water-soluble substances. To measure these, we use a logarithmic scale known as pH. Here we are discussing pH scale with examples.
Thus pH solution is the negative logarithm of hydrogen ion. Whenever the pH value of any solution is 7, then it will be a neutral solution.
Features of pH scale with examples
Below mentioned are some key features of the pH scale that one must know.
pH Value
The pH scale runs from the pH value of 0 to 14, which indicates how acidic or basic a substance is. Scientists usually use litmus paper to test the pH value. A natural water-soluble dye is used as a pH indicator.
Color of the Indicator
The indicator’s color changes from red for strongly acidic to dark purple for strongly alkaline. The dark red color indicates pH value one, and the violet color on the litmus paper indicates pH value 14. pH value 1 means the substance is acidic, while the pH value 14 implies the element is alkaline.
Neutral
pH seven is indicated by green color in the middle of the two portions. Generally, the pH value of pure water is 7. The neutral solutions hit seven on the pH scale. These solutions do not affect a litmus indicator or solution.
Acidic and Alkaline
The acidic solutions mark less than 7. The alkaline solutions characterize more than 7. Most of our body except stomach acid measure around 7.2 and 7.6 on the pH scale. That is why when a potent foreign substance comes in our body; our body can not function properly.
Molecule |
Found in |
pH |
Type |
pHospHoric acid |
fizzy drinks |
2,152,15 |
acid |
tartaric acid |
wine |
2,952,95 |
acid |
citric acid |
lemon juice |
3,143,14 |
acid |
acetic acid |
vinegar |
4,764,76 |
acid |
carbonic acid |
fizzy drinks |
6,376,37 |
acid |
ammonia |
cleaning products |
11,511,5 |
base |
ammonium hydroxide |
cleaning products |
11,6311,63 |
base |
sodium hydroxide |
caustic soda |
1313 |
base |
Increase in pH
The pH of acidic solutions increases towards the mark 0. Acid solutions having pH value two are stronger than solutions with pH value five. Similarly, the pH of alkaline solutions increases towards the mark 14.
Concentration of H+
The pH scale is a log scale. Any change in the pH value from 2 to 6 represents a high H+ concentration. In the pH, the pH value of any solution is numerically equal to the logarithm of the inverse of hydrogen ion (H+) concentration.